Gene Editing Market is Expected to Grow USD 22.87 Billion by 2032 | At CAGR 12.06%
The global gene editing market size was valued at USD 9.78 billion in 2024.
Integration of AI and Automation for Efficient Gene Editing Workflows is a Prominent Trend Observed ”
NY, UNITED STATES, February 10, 2026 /EINPresswire.com/ -- The global gene editing market size 2026 demonstrates substantial growth potential, having reached a valuation of USD 9.78 billion in 2024. Market projections indicate expansion to USD 10.31 billion in 2025, ultimately achieving USD 22.87 billion by 2032. This represents a compound annual growth rate of 12.06% throughout the forecast period, reflecting the increasing adoption of precision genetic modification technologies across therapeutic, research, and diagnostic applications.— Fortune Business Insights
Gene editing encompasses advanced technologies enabling precise DNA modifications within organisms. These tools find extensive applications in treating genetic disorders and developing diagnostic assays. The market expansion stems from continuous advancements in editing platforms including CRISPR, TALENs, and base editors, which enhance modification efficiency. Additionally, rising prevalence of congenital diseases, increased investment activity, and personalized medicine developments further accelerate market momentum.
Get a Free Sample PDF - https://www.fortunebusinessinsights.com/enquiry/request-sample-pdf/gene-editing-market-104463
Key Market Drivers
The surge in personalized medicine demand, coupled with expanding therapeutic pipelines, serves as the primary growth catalyst. The precision medicine focus intensifies through accelerated investments in CRISPR and alternative gene-editing platforms. These technologies facilitate precise genomic modifications, enabling curative therapies for conditions such as sickle cell disease, beta-thalassemia, and various rare genetic disorders.
Expanding clinical trials and pipeline candidates for these conditions significantly contribute to market advancement. Regulatory approvals reinforcing gene-edited therapy safety further propel growth. Notable developments include Vertex Pharmaceuticals' January 2024 authorization from Saudi Arabia's Food and Drug Authority for CASGEVY, a CRISPR/Cas9 gene-edited therapy treating sickle cell disease and transfusion-dependent beta thalassemia.
The Children's Hospital of Philadelphia and Penn Medicine demonstrated therapeutic effectiveness in May 2025 by successfully treating a child with severe carbamoyl phosphate synthetase 1 deficiency using customized CRISPR gene editing therapy, showcasing the technology's potential for rare genetic disorders.
Market Challenges and Restraints
Skilled workforce shortages represent a significant impediment to market expansion. Meeting increasing global demand requires trained professionals capable of executing complex workflows, including CRISPR operations, high-throughput sequencing systems, and GMP-grade manufacturing. Developing and commercializing gene-edited therapies demands specialized expertise in molecular biology, bioinformatics, genomic engineering, and regulatory compliance.
Significant infrastructure and technical training program gaps result in qualified researcher, technician, and research institute shortages. The U.S. Government Accountability Office reported in March 2023 concerning shortages in current and projected laboratory and biomanufacturing technicians supporting regenerative medicine and advanced therapy development.
Off-target effects pose additional challenges, as unintended genetic modifications at non-target sites may cause unwanted mutations, altered gene functions, or tumorigenic risks. These factors limit broader clinical adoption and slow commercialization. A July 2025 NIH study highlighted substantial off-target genotoxicity concerns delaying clinical translation of gene editing therapies.
Market Segmentation Analysis
By Offering
The products segment dominates the market with the largest share, driven by recurring consumption of kits, RNAs, nucleases, enzymes, transfection reagents, and critical reagents in workflows. New product launches compatible with large-scale production and automation drive growth. Strategic collaborations, such as Aldevron's partnership with Integrated DNA Technologies in May 2025, successfully manufactured personalized CRISPR gene editing drug products, demonstrating industry innovation. The services segment projects growth at 14.57% CAGR during the forecast period.
By Technology
CRISPR technology holds the largest market share, commanding 61.0% in 2025. This dominance stems from superior efficiency and cost-effectiveness compared to alternative genome editing tools. CRISPR offers higher editing efficiency, multiplexing capability, and compatibility with high-throughput workflows, reducing development time and costs. January 2025 witnessed EditCo Bio launching XDel Knockout Cells, revolutionizing CRISPR gene editing through groundbreaking guide RNA design strategy. TALENs segment projects 11.13% CAGR growth during the study period.
By Application
The therapeutic segment anticipates fastest growth over the forecast period, holding 26.5% share in 2025. This growth attributes to gene editing therapy effectiveness for various critical conditions. Rising global prevalence of congenital and inherited disorders increases demand for precise, targeted treatment options. Growing pharmaceutical and biotechnology company investments toward expanding clinical applications contribute to segmental advancement. The diagnostics segment projects 14.05% CAGR growth.
By End User
Pharmaceutical and biotechnology companies led the market in 2024, holding 45.3% share in 2025. These companies utilize gene editing products at large scale for therapeutic application development. Infrastructure advancement investments maximize commercialization capabilities. YolTech Therapeutics received USD 45 million funding from the AstraZeneca-CICC healthcare investment fund in September 2025 to support clinical program advancement. Research and academic institutes segment projects 10.36% CAGR growth.
Regional Market Analysis
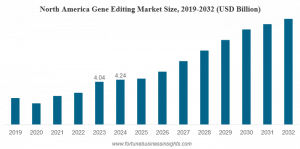
North America dominated the market in 2024 at USD 4.24 billion, attributed to robust infrastructure and increasing investment activities. Leading company presence and favorable government policies strengthen market dominance. Strategic collaborations among biotechnology and pharmaceutical companies drive regional growth. The U.S. market estimates reaching USD 2.03 billion in 2025.
Europe projects 11.82% growth rate, reaching USD 2.68 billion by 2025. Growth stems from rising precision medicine demand and favorable government policies. The U.K. anticipates USD 0.56 billion valuation, Germany USD 0.64 billion, and France USD 0.51 billion in 2025.
Asia Pacific estimates reaching USD 2.01 billion in 2025, securing third-largest regional position. China and India both estimate reaching USD 0.55 billion and USD 0.29 billion respectively in 2025. Government-pharmaceutical company collaboration increases drive regional growth.
Latin America market sets to reach USD 0.60 billion in 2025. Academic collaboration increases and rising genomic medicine awareness drive market growth. In Middle East and Africa, the GCC sets to reach USD 0.23 billion by 2025.
Speak To Analyst- https://www.fortunebusinessinsights.com/enquiry/speak-to-analyst/gene-editing-market-104463
Competitive Landscape and Industry Developments
The market exhibits semi-consolidated structure, comprising prominent players including Thermo Fisher Scientific Inc., Agilent Technologies Inc., and GenScript. These companies hold significant market share through numerous new product launches, acquisitions, technological advancements, and strategic activities.
Agilent Technologies acquired BIOVECTRA in July 2024 for USD 925 million, expanding CDMO specialization in oligonucleotides and CRISPR therapeutics. November 2025 witnessed SOHM Inc. receiving FDA commentary and industry analyses signaling regulatory pathway evolution for genome-editing technologies.
Ashwin Arora
Fortune Business Insights™ Pvt. Ltd.
+1 833-909-2966
sales@fortunebusinessinsights.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.