Global Zero Static Diaphragm Valve Market Growth 2026–2036 Driven by Process Purity and Validation Standards
Regulated manufacturing and contamination control priorities push the zero static diaphragm valve market toward steady long-term growth.
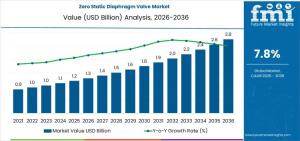
NEWARK, DE, UNITED STATES, January 28, 2026 /EINPresswire.com/ -- The Zero Static Diaphragm Valve Market is positioned for sustained expansion as pharmaceutical, biotechnology, and high-purity chemical manufacturers intensify efforts to eliminate contamination risk across fluid transfer systems. Global market value is estimated at USD 1.3 billion in 2026 and is forecast to reach USD 2.8 billion by 2036, reflecting a 7.8% CAGR. Growth is anchored in process qualification cycles and facility buildouts rather than short-term maintenance spending.
Request For Sample Report | Customize Report | Purchase Full Report –
https://www.futuremarketinsights.com/reports/sample/rep-gb-31363
Unlike conventional valves, zero static diaphragm valves are embedded into process layouts at the design stage. Their primary role is to remove dead legs where product residue can accumulate and compromise sterility or batch yield. Once validated, these components are rarely replaced without triggering extensive requalification, making early specification decisions critical. As a result, market demand closely follows new line installations, capacity expansions, and major process reconfiguration projects.
Market Size and Growth Outlook 2026–2036
The market’s growth trajectory reflects the steady build-out of regulated production capacity worldwide. Demand is strongest in pharmaceutical and biotech manufacturing, where contamination events directly impact compliance status and revenue.
Key growth indicators include:
- Market value of USD 1.3 billion in 2026
- Forecast value of USD 2.8 billion by 2036
- CAGR of 7.8% over the forecast period
- Expansion led by Asia Pacific, Europe, and North America
Rather than rapid replacement cycles, expansion comes from additional process steps, higher segregation of fluid paths, and stricter internal cleanliness standards.
How Zero Static Diaphragm Valves Enable Contamination Control
Zero static diaphragm valves are designed so that no hold-up volume remains once the valve closes. This feature allows complete drainage and repeatable clean-in-place cycles, which are essential in sterile and hygienic processing environments.
Performance is evaluated through:
- Drainability and dead-leg elimination
- Surface finish stability
- Diaphragm life under repeated cleaning cycles
- Leak integrity and pressure performance
Engineers specify these valves by geometry and cleanability rather than size alone, positioning them as process-critical components rather than commodity hardware.
Demand Drivers Shaping the Market in 2026
Specification in the zero static diaphragm valve market is governed by validation and change-control frameworks. Once a valve type becomes part of approved piping and instrumentation documentation, changes are avoided to prevent revalidation.
Primary demand drivers include:
- New pharmaceutical and biotech facility construction
- Clean utility and process line upgrades
- Increased segregation of critical fluid paths
- Tighter audit and inspection expectations
These factors tie market momentum to capital project pipelines rather than routine operational budgets.
Valve Type Trends and Compliance Impact
Zero static diaphragm valves account for approximately 46% of total demand due to their alignment with sterile and high-purity process requirements. Other diaphragm valve types address varying levels of hygiene and automation needs.
Valve type dynamics:
- Zero static diaphragm valves dominate sterile applications
- Sanitary diaphragm valves serve high-hygiene, lower sterility uses
- Pneumatic diaphragm valves support automated control systems
- Manual diaphragm valves remain in auxiliary and utility services
Once validated, valve type selection establishes long-term inspection, maintenance, and documentation obligations.
End Use Industry Influence on Market Structure
Pharmaceutical and biotech manufacturers represent around 44% of total demand, driven by stringent regulatory oversight and high contamination risk sensitivity. Other industries contribute volume with varying specification rigor.
End-use segmentation highlights:
- Pharmaceuticals and biotech lead in value and specification depth
- Food and beverage emphasizes hygiene with moderate validation
- Chemicals prioritize material compatibility and containment
- Water and wastewater focus on volume with lower hygienic thresholds
This structure concentrates high-value demand in life sciences while supporting broader adoption elsewhere.
Regional Growth Patterns
Asia Pacific shows the fastest growth, led by India and China. India records a projected 9.0% CAGR, supported by sterile drug manufacturing and vaccine production. China follows at 8.5%, driven by biologics, semiconductor chemicals, and regulated industrial projects. The United States grows at 7.2% through compliance-driven retrofits, while Europe reflects steady modernization cycles.
Competitive Landscape and Supplier Positioning
Competition centers on early-stage process approval rather than price-based replacement sales. Suppliers such as Emerson, Swagelok Company, ITT Inc., and KSB SE & Co. KGaA compete through documentation quality, surface finish control, and lifecycle reliability.
Competitive success depends on:
- Inclusion in approved equipment lists
- Consistent manufacturing and testing standards
- Reliable delivery aligned with project schedules
- Strong technical and validation support
Once approved, supplier positions are reinforced by long-term process governance rules.
Market Outlook
The zero static diaphragm valve market will continue to grow as contamination control becomes non-negotiable in regulated manufacturing. Skid-based modular construction and repeatable line designs further reinforce demand consistency. However, higher unit costs and qualification burdens will keep adoption focused on high-purity and high-risk applications.
Get data that aligns with your strategic priorities — ask for report customization today:
https://www.futuremarketinsights.com/customization-available/rep-gb-31363
Related Reports:
Industrial Planetary Gearbox Market- https://www.futuremarketinsights.com/reports/industrial-planetary-gearbox-market
Instrumentation Valve and Fitting Market- https://www.futuremarketinsights.com/reports/instrumentation-valve-and-fitting-market
India Solar Panel Mounting Structure Market- https://www.futuremarketinsights.com/reports/industry-analysis-of-solar-panel-mounting-structure-in-india
Have a specific Requirements and Need Assistant on Report Pricing or Limited Budget please contact us - sales@futuremarketinsights.com
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware - 19713, USA
T: +1-347-918-3531
Why FMI: https://www.futuremarketinsights.com/why-fmi
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.